Ice CrystalsOn November 4-5, 2006, overnight temperatures in North Salem, NY fell to the dew point and resulted in the deposition of a very light frost on the freshly waxed surface of my car. Closer examination revealed several great illustrations of "chemical siphoning"* of water molecules from smaller crystals to adjacent larger ones. Notice in the detail photo that while the surface is generally covered with a very light frost, there are 3 larger needle-like ice crystals growing out from a central point (likely a speck of dust that served as a deposition nucleus), and that the area immediately adjacent to the larger crystals is completely free of ice! The entire roof and trunk were peppered with these little structures. 
It's harder for a water molecule to leave a larger crystal than it is to leave a smaller crystal, so the saturation vapor pressure over the larger crystal is lower than it is over the smaller frost crystals. Over time, then, there is net deposition on the larger crystals and net sublimation of the smaller adjacent crystals - water molecules are 'siphoned' off of the small crystals and deposited onto the larger ones. Notice that the siphon effect is least near the very narrow tip of the upper right needle. It's also interesting to note that the middle ice needle is growing up from the surface of the car - if you look carefully you can see the small shadow it's casting. 
Growth of liquid water droplets in clouds can occur in a similar way - larger droplets (with a lower saturation vapor pressure around them) grow at the expense of smaller water droplets around them. And in a cloud that contains both liquid water droplets and ice crystals, the ice crystals quickly take up and hang onto the water molecules that find it easier to leave the liquid droplets - in a short time only ice crystals exist in the cloud. 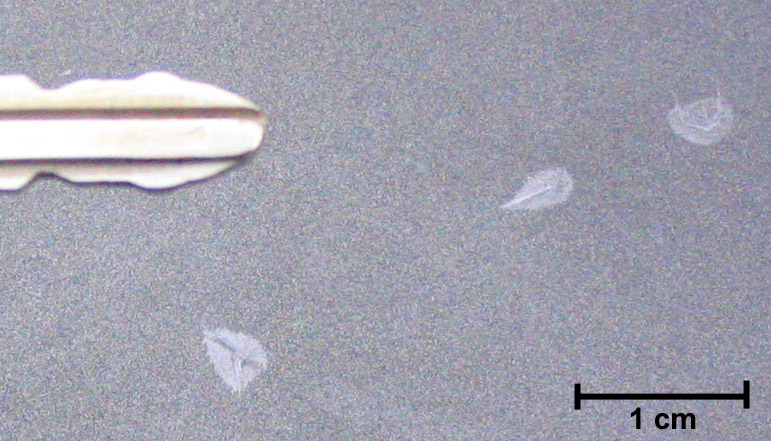
The photo above was taken a few weeks later, when I noticed the ice crystals that had grown overnight on the windsheild of my car. The photo was taken from inside the car. This chemical siphoning occurs as metamorphic rocks form, too, resulting, for instance, in the growth of large garnet crystals within a fine grained mica schist, in the metamorphosis of fine grained slate to phyllite to schist, and in the transformation of limestone to marble. * Thanks to Syracuse U. Geology Professor Emeritus Dr. Bryce Hand for the term "chemical siphon", a term I first heard in a discussion with Bryce regarding metamorphic processes. His comments from recent email correspondance regarding chemical siphoning follow: Well, I don't know whether [the term "chemical siphon"] derives from some long-forgotten source or whether it's as original as I've always assumed. As far as I'm concerned, it's an excellent descriptor. Energetically, the principle is absolutely the same as for a fluid siphon...or a Slinky. It's not just a name; it actually describes the phenomenon. The other chemical siphon I alluded to was a demo in an empty instant-coffee jar that I began one day after lecturing on the importance of relative humidity as a control on the "highest" (i.e., most soluble) salt in an evaporite deposit. I put two 50-ml beakers in the jar — one half full of tap water, the other half full of salty water — screwed on the cap, and let the thing sit on a shelf in my office. I'd remove and weigh the beakers every week or so. Over time, the amount of fresh water declined, and the salt water increased until, after a couple months all water had been transferred to the salty beaker. I suspect it would have gone to completion much faster if I'd used a mechanical stirrer, rather than relying on diffusion to mix the continually-freshened surface layer into the bulk of salt water. Another time I'd use the term was in explaining diagenesis of lime sediments. On the blackboard, I'd draw two mineral chunks in a beaker of water. I'd label one aragonite (maybe a clam shell) and the other calcite (say, a crinoid ossicle). Then I'd explain that both solids would release CaCO3 to the solution in an attempt to reach equilibrium. But as the highly soluble aragonite tried to saturate the solution with respect to itself, the concentration would pass the equilibrium solubility of calcite. Precipitation of calcite would then remove solute from the supersaturated (with respect to calcite) solution, while the aragonite would continually add more to the undersaturated (with respect to aragonite) solution. Eventually: no aragonite, just calcite. In terms of limestone diagenesis, this likely would mean an empty mold where the clam had been, and a large calcite overgrowth (calcite cement) on the crinoid. I couldn't put numbers on the calcite/aragonite example, because so many factors affect the solubilities. But with chert, I'd start by drawing two beakers of water...one with biogenic opal (the original stuff of diatoms, radiolarians, etc.) and the other with quartz. I'd write 120 ppm for the equilibrium concentration for opal, and 6 ppm for quartz. Then I'd reconfigure the setup so both solids were in a single container...and continue as for CaCO3. Bryce Comments to Steve Kluge |